Services
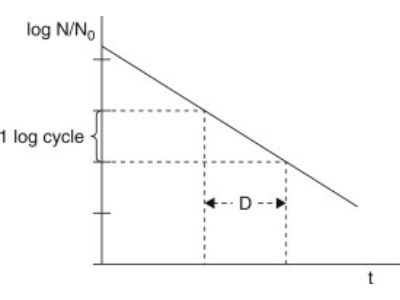
1. D-value and Z-value determination
Liofilchem performs D-Value and Z-Value analysis by direct inoculation of product or equipment using Biological Indicator Organisms (BI's).
The test is mainly used to verify the labeled D-value of BIs to confirm their suitability for use and to determine the resistance of inoculated products,
process challenge devices (PCDs), isolates recovered from bioburden tests, environmental monitoring, product sterility tests.
D-values are stated by the manufacturer on the BIs but often require verification for a higher degree of accuracy and process specificity.
SAL should always be established in relation to a D-value that is higher than that of the natural bioburden at routine production.

Our Biological Indicator Evaluation Resistometer (BIER) Vessel autoclave verifies the Dvalue by accurately delivering a calibrated and pre-determined level of lethality, such as a steam sterilisation at 121°C, 118°C and 132°C.
The log reduction achieved for exposure time is assessed through the exposure of the BI for defined time points and interpretation via fractional analysis.
This process allows the accurate verification of the manufacturer’s stated D-value.
The use of appropriate biological indicators, with a D-value that is representative of (and not lower than) the thermal resistance of the indigenous environmental bioburden, is a key element of the Contamination Control Strategy (CCS) and supports the demonstration that a validated sterilisation process achieves the required Sterility Assurance Level (SAL).
Bioburden characterization became mandatory requirement when the overkill approach is not used (refer to EMA CPMP 2015).
Equipment & Method
The design of the BIER vessel, the maintenance schedule, and the utilities employed are fully aligned with pharmaceutical industry standards. This ensures the precise and consistently reproducible determination of microbial resistance, closely simulating the actual conditions under which the biological indicator will be used by the customer. Such rigour delivers not only scientific accuracy but also tangible value for clients seeking the highest reliability in their sterilisation validation processes.
- Equipment
D value is performed in an internal laboratory
certified by TÜV Süd to ISO 9001 for the quality
management. The BIER vessel is a Fedegari
FOB series design compliant to ANSI/AAMI/
ISO 18472 requirements Sterilization of Health
Care Product - Biological and Chemical
Indicators and designed to create precise and
repeatable sterilizing environments, allowing
the evaluation of their effect on biological
inactivation kinetics, chemical reactions,
material degradation and product bioburden.
BIER Resistometer allows precise variation of
the environmental conditions and cycle
sequences in order to produce controlled
physical studies.
- Measurement Accuracy (Maintenance, Calibration, Monitoring)
- The probes used to measure temperature and pressure from within the steam resistometer are annually certified.
- Re-qualification of the equipment is performed annually.
- Purified water system subjected to biweekly chemical and microbiological checks.
- Steam meets the requirements of the Non-Condensable Gases (NCG) test is compliant with steam quality standards necessary for moist-heat sterilization.
- A preventive maintenance plan for the equipment’s critical components is in place.
- Method
Stumbo-Murphy-Cochran, Holcomb-Spearman-Karber, and Limited HolcombSpearman-Karber methods could be used according to ANSI/AAMI/ISO 11138.
2. Biological Indicator Population - Total Viable Spore Count
Population Verification (Spore Counts) is performed before the D-value determination to
confirm that the microbial population within a batch of BIs matches the manufacturer’s
Certificate of Analysis.
Heat Shocked and Non-Heat Shocked, Pooled or Individual BIs is verified according to
USP or ISO 11138-1- Sterilization of health care products- Biological Indicators.
3. Customized biological indicators
Customized BIs are essential in situations where a standard BI would not provide an
adequate challenge to the sterilization process, they may not accurately mimic the specific
size, shape, and material of a unique product.
Products like vial stoppers i.e. present sterilization challenges due to their various
configurations and materials, requiring a custom BI for a truly accurate result.
Liofilchem is available to prepare ad hoc BIs according to customers’ requirements.
In this application, using the D-value to determine resistance is essential for certifying the
biological indicator and ensuring compliance with ISO standards and regulatory
requirements.
4. Fit-for-purpose studies
Further studies may be required to deepen understanding of biological indicators and
products, and to enhance internal processes. For example, BI transport qualification
confirms retention of population characteristics and resistance during shipping, in
accordance with ISO 11138-7.
Liofilchem can support your company with these studies, aligned with to your needs.

References
European Pharmacopoeia (Ph. Eur.) 10th Edition, Biological Indicators of Sterilization.
US Pharmacopeia, current version, USP–NF 2021, Biological Indicators Resistance Performance Test.
ISO 11138-1:2017 Sterilization of health care products — Biological indicators — Part 1: General requirements.
ISO 11138-2:2017 Sterilization of health care products — Biological indicators — Part 2: Biological indicators for ethylene oxide sterilization processes.
ISO 11138-3:2017 Sterilization of health care products — Biological indicators — Part 3: Biological indicators for moist heat sterilization processes.
ISO 11138-4:2017 Sterilization of health care products — Biological indicators — Part 4: Biological indicators for dry heat sterilization processes.
ISO 11138-7:2017 Sterilization of health care products — Biological indicators — Part 7: Guidance for the selection, use interpretation of results.
European Medicines Agency (EMA). Guideline on the sterilisation of the medicinal product, active substance, excipient and primary container. EMA/CHMP/CVMP/QWP/850374/2015

