A.F. GENITAL SYSTEM (ref. 74156) is a 24 well panel containing biochemical substrata and antimicrobial drugs for detection, presumptive identification and susceptibility testing of microorganisms from urogenital specimens (vaginal swab, urethral swab, seminal fluid, urine).
A.F. GENITAL SYSTEM is inoculated with the suspension of the clinical specimen and incubated at 36 ± 1 °C for 24 hours.
Test results are conveniently read by evaluating color changes in the wells.
A.F. Genital System delivers four types of results in one panel:
- Count of urogenital mycoplasma
- Identification of Mycoplasma hominis and Ureaplasma spp.
- Susceptibility testing of urogenital mycoplasma
- Bacterial and fungal screening
Identification and enumeration of Ureaplasma spp. and Mycoplasma hominis
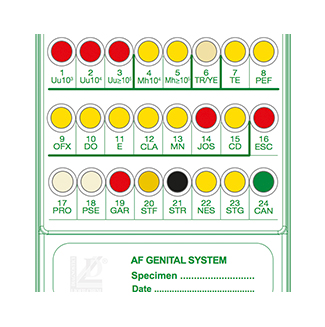
Count of Ureaplasma spp. (Uu) 10e3 to ? 10e5 CFU/mL
Count of Mycoplasma hominis (Mh) 10e4 to ? 10e5 CFU/mL
Susceptibility testing of urogenital mycoplasmas
Growth is indicated by yellow to red color change in wells 7 to 15.


Bacterial and fungal screening
Interpretation by clear color change in the wells:
- Escherichia coli
- Proteus spp. / Providencia spp.
- Pseudomonas spp.
- Gardnerella vaginalis
- Staphylococcus aureus
- Enterococcus faecalis
- Neisseria gonorrhoeae
- Streptococcus agalactiae (Group B)
- Candida spp.
Just like all the Liofilchem® microbiological identification systems, the A.F. Genital System has the great benefits of:
- performing simultaneous microbiological screening of several microorganisms in one compact panel;
- reducing costs in man hours and laboratory consumables;
- requiring no complex lab instruments because of its extraordinary ease of use.
The wide range microbiological screening of the Liofilchem® identification systems permits to obtain a complete overview of the microbial population in the specimen, with the possibility to carry out successive confirmation analysis only for the microorganisms resulted positive at the system.
Biochemical, immunoserological and microscopic confirmation tests or susceptibility test following the screening can be started just by taking a drop of culture broth from the well of the microorganism to be confirmed.

