Compact Antimicrobial Susceptibility Panel
for determining MIC through broth microdilution according to ISO 20776-1:2019 standard
Broth microdilution is used as a reference method for AST for leading therapeutic decisions. Liofilchem developed its Broth microdilution range on a proprietary compact format, in addition to a large AST portfolio that includes MIC Test Strip, disks and ready-to-use agar dilution panels.
ComASP® delivers real MIC results for antimicrobial agents that are indicated by the international guidelines to be used in broth microdilution rather than agar diffusion, agar dilution or automated/semi-automated methods. ComASP® is also available for customizable assortments of agents and concentration ranges, to meet special needs of laboratories of any sizes carrying out antimicrobial susceptibility tests.
Incubation at 36±2°C for 16-20 hours in ambient air.
At the end of the incubation period watch for the growth in the wells and determine the MIC, i.e. the lowest concentration of antibiotic that inhibits visible growth.
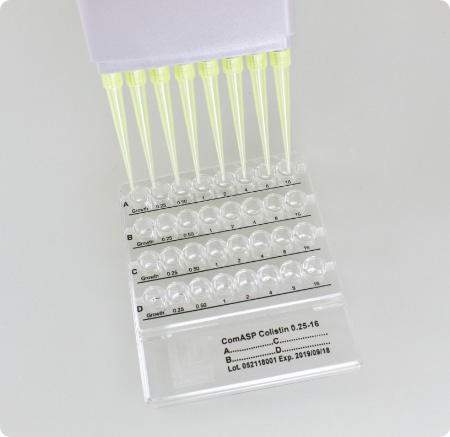

Growth appears as turbidity or as a button at the bottom of the well (compare with the amount of growth in the growth-control well). The result can be read visually or automatically. Reading by the naked eye can be improved by use of bright indirect lighting against a dark background
CE marked. Available in Europe as IVD.
Click here for the complete ComASP® range
References
Comparison of commercial methods for evaluating susceptibility to cefiderocol.
P. Pini, R. Marrollo, P. Nardini, C. Bedogni, R. Bianchi, R. Leo, V. Manghi, E. Carretto
ECCMID 2024
blaVIM Carrying Pseudomonas aeruginosa. The Antimicrobial Resistance Nightmare with Few Therapeutic Options.
J. Alexander, D. Navas, A. Charles, W. Merced
Multivariable Evaluation of In-Vitro Performance of Ceftolozane/Tazobactam, Ceftazidime/Avibactam, Imipenem/Relebactam & Cefiderocol on Difficult-to-Treat Pseudomonas aeruginosa Isolated from Clinical Samples
J. Alexander, MD. Director, Clinical Microbiologist. AdventHealth Orlando, Florida
ECCMID 2024
Is there another option other than Daptomycin for Difficult-To-Treat Vancomycin-Resistant Enterococcus faecium. In-Vitro Activity of Telavancin, Dalbavancin and Oritavancin..
T. Gordon; D. Navas; J. Alexander; A. Charles
In-Vitro Activity of Cefiderocol Against Multidrug Resistant Strains of P. aeruginosa DTR, A. baumanni complex DTR, A. xylosoxidans MDR, S. maltophilia MDR & B. cepacia complex, by Iron-Depleted Broth Microdilution.
J. Alexander, D. Navas, A. Charles
IDWeek 2023, Poster #2745
Disc Diffusion and ComASP® Cefiderocol Microdilution Panel to Overcome the Challenge of Cefiderocol Susceptibility Testing in Clinical Laboratory Routine
G. Bianco, M. Boattini, S. Comini, G. Banche, R. Cavallo, C. Costa
Antibiotics 2023, 12, 604
In-Vitro Evaluation & Validation of the Custom Liofilchem® Broth Microdilution Synergy Panel Containing Aztreonam Plus Ceftazidime/Avibactam for Clinical Use.
Daniel Navas, MLS, Jose Alexander, M.D., Angela Charles, MLS
ASM Microbe 2022
Evaluation of ComASP oritavancin test for rapid antibiotic susceptibility testing of Gram-positive clinical isolates
L.M. Lazzaro, M. Cassisi, S. Stefani, F. Campanile
ESCMID eLearning. 07/09/21; 328476; 1985
The accuracy of four commercial broth microdilution tests in the determination of the minimum inhibitory concentration of colistin.
Yusuf, E., van Westreenen, M., Goessens, W. et al.
Ann Clin Microbiol Antimicrob 19, 42 (2020)
Comparative Evaluation of CHROMagar COL-APSE, MicroScan Walkaway, ComASP Colistin, and Colistin MAC Test in Detecting Colistin-resistant Gram-Negative Bacteria.
Osei Sekyere, J., Sephofane, A.K. & Mbelle, N.M.
Sci Rep 10, 6221 (2020)
Antimicrobial Susceptibility testing of Enterococcus spp. in a VanB-VRE outbreak setting
P. C. Lindemann, T. S. Haukeland, H. Kolstad, B. C. Haldorsen, K. Hegstad
NSCMID 2019
Evaluation of three methodologies for in vitro susceptibility testing of Ceftolozane Tazobacatam (C/T)
E. Widlake, TR Walsh, JM. Tyrrell
FEMS 2019
A comparison of Piperacillin-Tazobactam and Colistin ComASP® (SensiTest) MIC to CLSI Broth Microdilution MIC for Gram Negative Challenge Isolates
L. Koeth, J. DiFranco-Fisher Lab. Specialists, Inc., Westlake, OH
ASM Microbe 2018
Evaluation of ComASP® (SensiTest) Colistin, a commercial broth microdilution-based method to evaluate colistin MICs for Klebsiella pneumoniae isolates
Irene Galani, Panagiota Adamou, Ilias Karaiskos, Helen Giamarellou, MariaSouli
ECCMID 2018
Clinical Validation of ComASP® (SensiTest) Colistin, a Broth Microdilution-Based Method To Evaluate Colistin MICs
Edoardo Carretto, Flavia Brovarone, Giuseppe Russello, Paola Nardini, Maisra M. El-Bouseary, Ali F. Aboklaish, Timothy R. Walsh, Jonathan M. Tyrrell
DOI: 10.1128/JCM.01523-17
Preliminary evaluation of the ComASP® (SensiTest) Colistin, a broth microdilution based method to evaluate colistin MICs
Edoardo Carretto, Flavia Brovarone, Mariagrazia Sciascia, Giuseppe Russello
ECCMID 2017

