Featured products
Antibiotic Discs
View the complete range of antibiotic discs in cartridges
View the complete range of antibiotic discs in canister packaging
Technical sheets
| Antibiotic discs | |
| Antibiotic Disc: Instructions for Use | rev.18 - 20/07/2023 |
| Antibiotic Disc Interpretative Criteria and Quality Control | rev.18 - 20/07/2023 |
| Cefiderocol 30 mcg disc | rev.1.1 - 12/05/2021 |
| Oritavancin 5 mcg disc | rev.1 - 22/03/2022 |
| Non-hazardous Product Statement Antibiotic-Antifungal Disc | rev.2 - 17/04/2023 |
| CE Declaration of Conformity Antibiotic-Antifungal Disc | rev.37.4 - 22/05/2023 |
| Antifungal discs | |
| Antifungal disc: Instructions for Use | rev.9 - 02/07/2021 |
| Non-hazardous Product Statement Antibiotic-Antifungal Disc | rev.2 - 17/04/2023 |
| Multodiscs | |
| Multodisc: Instructions for Use | rev.1.1 - 17/06/2021 |
| VETMultodisc | rev.0 - 04/02/2020 |
| Non-hazardous Product Statement Multodiscs | rev.0 - 21/03/2018 |
| Disc kits for resistance mechanisms detection | |
| 99002 EUCAST ESBL disc kit | rev.1 - 06/02/2024 |
| 99003 KPC&MBL disc kit (acc. to EUCAST) | rev.2.2 - 29/07/2016 |
| 99004 ESBL disc kit (acc. to EUCAST) | rev.1 - 06/02/2024 |
| 99005 ESBL disc kit (acc. to CLSI) | rev.0.1 - 31/08/2015 |
| 99006 ESBL (Chromos. Ind. AmpC) disc kit (acc. to EUCAST) | rev.1 - 06/02/2024 |
| 99007 KPC&MBL&OXA-48 disc kit (acc. to EUCAST) | rev.1 - 17/06/2016 |
| 99008 ESBL+AmpC screen disc kit | rev.0.1 - 31/08/2015 |
| 99009 AmpC disc kit | rev.0.1 - 08/09/2015 |
| ESBL disc tests | rev.2 - 19/06/2014 |
| Non-hazardous Product Statement Disc Kits for Antimicrobial Resistance Testing | rev.0 - 14/12/2018 |
| Brochure | |
| Lot specific e-IFU |
Accessories for antimicrobial susceptibility testing
Rotating Plate Inoculator for confluent microbial growth

D-value verification
Liofilchem performs D-Value and Z-Value analysis by direct inoculation of product or equipment using Biological Indicator Organisms (BI's).

D-values are stated by the manufacturer on the Biological indicator but often require verification for a higher degree of accuracy and process specificity. The Pharmacopoeias and ISO11138 stress on the importance of D-value verification to ensure the accuracy of a stated manufacturer D-value.
D-value verification should be performed if a biological indicator bioburden approach is used for sterilisation validation.
The D-value is the time taken at a given temperature to reduce the population of exposed microorganisms by 90% or achieve a 1-log reduction.
The Z-value is the temperature increase required to reduce the d-value by one log.
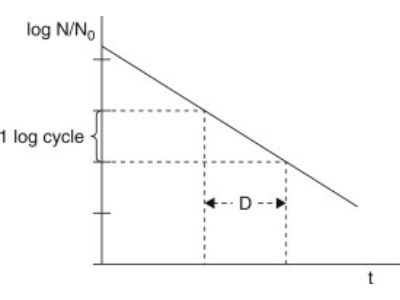
For example, D-value of 2.5 minutes at 121°C equates to a 90% reduction in viable bacteria in 2.5 minutes at those conditions. If the temperature is increased to 131°C and the D-value at 131 is 0.25 minutes – the Z-value can be said to be 10°C.
Our Biological Indicator Evaluation Resistometer (BIER) Vessel autoclave verifies the D-value by accurately delivering a calibrated and pre-determined level of lethality, such as a steam sterilisation at 121°C. The effect of the stable sterilisation conditions on biological indicator resistance with the heat up and cool down phase having minimal contribution to the Fo is assessed.
The log reduction achieved for exposure time is assessed through the exposure of the BI for defined time points and interpretation via fractional analysis. This process allows the accurate verification of the manufacturer’s stated D-value.
A high Sterility Assurance Level can only be achieved by an accurate D-value calculation.

References
European Pharmacopoeia (Ph. Eur.) 10th Edition, Biological Indicators of Sterilization.
US Pharmacopeia, current version, USP–NF 2021, Biological Indicators Resistance Performance Test.
ISO 11138-1:2017 Sterilization of health care products — Biological indicators — Part 1: General requirements.
ISO 11138-2:2017 Sterilization of health care products — Biological indicators — Part 2: Biological indicators for ethylene oxide sterilization processes.
ISO 11138-3:2017 Sterilization of health care products — Biological indicators — Part 3: Biological indicators for moist heat sterilization processes.
ISO 11138-4:2017 Sterilization of health care products — Biological indicators — Part 4: Biological indicators for dry heat sterilization processes.